The situation 情況
The bottle: Friend & foe in bioprocessing 瓶子:生物加工中的朋友和敵人
Despite the growing shift toward single-use bags, sterile bottles remain a staple in many biopharmaceutical workflows, such as
盡管人們越來越多地轉向使用一次性袋子,但無菌瓶仍然是許多生物制藥工作流程中的主要組成部分,例如
Early-stage development 早期開發
Small-volume fills 小容量分裝
Stability studies 穩定性研究
Buffer, media, solution addition 緩沖液、培養基、溶液添加
Their rigid structure and compatibility with existing lab infrastructure make them a reliable choice in specific scenarios.
它們的堅固結構和與現有實驗室基礎設施的兼容性使其成為特定場景中的可靠選擇。
Bottles present limitations in modern bioprocessing: Fluid management utilizing bottles is less scalable and difficult to automate. Additionally, bottles pose contamination risks due to open handling while also delivering inconsistent freezing results.
在現代生物工藝中,瓶子的使用存在諸多限制:使用瓶子進行液體管理的可擴展性較差,且難以實現自動化。此外,瓶子由于采用開放式操作,存在污染風險,并且會導致冷凍結果不一致。
However, advanced bottle filling technologies can turn this “foe" into a trusted “friend", tackling these challenges with precision and sterility.
然而,先進的瓶裝技術可以將這個“敵人"變成值得信賴的“朋友",以精準和無菌的方式應對這些挑戰。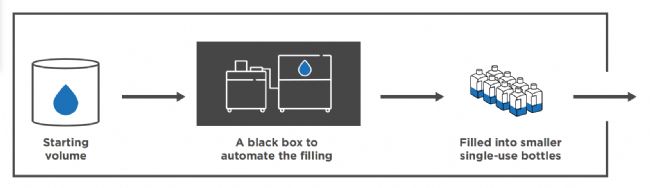
Case: Media filling into single-use bottles and 3D bags 案例:填充至一次性瓶和3D袋中
A biopharmaceutical manufacturer based in Latin America specializes in the production of liveattenuated vaccines. The upstream processing (USP) of these vaccines focuses on highdensity cell cultures, which are later used for virus activation and amplification. For these bioprocessing steps in cell expansion and virus propagation it is required to use media and buffer solutions.
一家位于拉丁美洲的生物制藥制造商專門生產減毒活疫苗。這些疫苗的上游加工(USP)側重于培養高密度細胞,隨后用于病毒的激活和擴增。在細胞擴增和病毒增殖的這些生物加工步驟中,需要使用培養基和緩沖液。
To maintain sterility and process flexibility, the company uses single-use bottles and 3D bags for aseptic media filling. The process accommodates a wide range of container formats – from bottles of 30mL to 2000mL, to large 3D single-use bags ranging from 50L to 200L.
為了保持無菌性和工藝靈活性,該公司采用一次性瓶和3D袋進行無菌培養基分裝。該工藝適用于各種容器規格——從30毫升到2000毫升的瓶子,到50升到200升的大型3D一次性袋。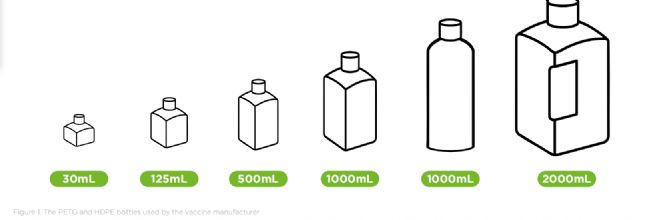
The challenge 挑戰
Complex media filling operations 復雜的培養基分裝操作
Due to the large variety and quantity of containers – up to 20 single-use bottles and 3D bags per batch – the manufacturer faces significant operational challenges.
由于容器種類繁多、數量龐大——每批次多達20個一次性瓶和3D包裝袋——制造商面臨著巨大的運營挑戰。
This complexity reduces efficiency and limits flexibility, particularly when adapting to varying batch sizes or container formats. Manual filling in a biosafety cabinet or isolator carries significant safety risks, raising the chance of contamination and jeopardizing product sterility. Together, these factors impede scalability and process robustness—making automation and closed-system solutions ever more essential.
這種復雜性降低了效率,并限制了靈活性,尤其是在適應不同的批次大小或容器規格時。在生物安全柜或隔離器中手動分裝存在重大安全風險,會增加污染的可能性,并危及產品的無菌性。這些因素共同阻礙了可擴展性和工藝的穩健性——這使得自動化和封閉系統解決方案變得更加重要。

The solution 解決方案
Making bottle filling a closed system 使瓶子分裝成為一個封閉的系統
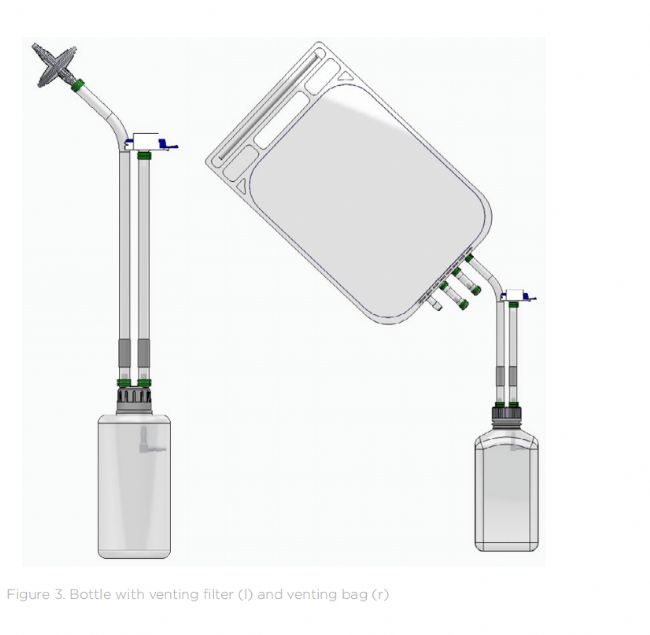
In aseptic manufacturing, converting bottle filling into a fully closed system is a key step in preserving product sterility across the entire fluid management process. This is achieved by using bottle caps equipped with sterile venting filters or venting bags that allow for pressure equalization during automated filling without exposing the product to the environment. Customizable singleuse bottle assemblies can be specifically designed to integrate with automated bottle filling systems, eliminating the need for manual intervention. The result: reduced contamination risk, assured GMP compliance (including Annex 1), and less reliance on high-grade cleanroom facilities.
在無菌生產中,將瓶裝分裝系統轉換為全封閉系統是在整個流體管理過程中保持產品無菌的關鍵步驟。這可以通過使用配備無菌排氣過濾器或排氣袋的瓶蓋來實現,這些過濾器或排氣袋可以在自動分裝過程中保持壓力平衡,而不會將產品暴露在環境中。可定制的一次性瓶組件可以專門設計用于與自動瓶裝分裝系統集成,從而無需人工干預。結果:降低污染風險,確保符合GMP標準(包括附錄1),并減少對高級潔凈室設施的依賴。
One size fills all: Automated & precise bottle filling with RoSS.FILL 一種尺寸適合所有情況:RoSS.FILL 自動精準分裝
Accommodating a wide range of container types and volumes, RoSS.FILL Bottle delivers a highly flexible and scalable solution for automated filling of both bottles and 3D single-use bags. Engineered for manufacturing cleanrooms, the system supports closed-system filling, ensuring precise, pressure-balanced operation and batch consistency for GMPcompliant dispensing.
RoSS.FILL Bottle 適用于各種類型和容量的容器,為瓶子和 3D 一次性包裝袋的自動分裝提供了高度靈活且可擴展的解決方案。該系統專為潔凈室制造而設計,支持封閉式系統分裝,確保精準、壓力平衡的運行和批次一致性,從而符合 GMP 要求。
Its modular design enables seamless setup changes, making it ideal for multiproduct facilities and dynamic production needs. The vaccine manufacturer was now able to use a setup with different racks connected to a single control unit to fill large volumes of media and buffer solution. This made it possible to fill both bottles and bags using the same system, tailored to the required packaging format (see figure 4).
其模塊化設計可實現無縫設置更改,使其成為多產品設施和動態生產需求的理想選擇。現在,該疫苗制造商能夠使用連接到單個控制單元的不同支架的裝置來分裝大量培養基和緩沖液。這使得能夠使用同一系統同時分裝瓶子和包裝袋,并根據所需的包裝規格進行定制(見圖 4)。

The result 結果
Being able to fill bottles of varying sizes and volumes has markedly improved operational efficiency over manual methods. Specifically, the vaccine manufacturer reported the following benefits for bottle filling:
與手動方法相比,能夠分裝不同尺寸和容量的瓶子顯著提高了操作效率。具體來說,疫苗制造商報告了瓶子分裝的以下優勢:
More than 3 times faster filling 分裝速度提高 3 倍以上
Including preparation, filling, and documentation in a sterile environment, the manual process averages 5 hours, while automation completes the task in just over 1 hour. Dispensing 150 liters into 96 single-use 2-liter bottles was thus approximately 70% faster with RoSS.FILL than with manual handling.
包括在無菌環境中的準備、分裝和記錄,手動流程平均耗時 5 小時,而自動化流程僅需 1 個多小時即可完成。因此,使用 RoSS.FILL 將 150 升液體分裝到 96 個 2 升一次性瓶中,比手動操作快約 70%。
Only 1 operator needed 僅需 1 名操作員
Including material handing and quality control, a standard manual filling process requires two to three operators, whereas automated filling machines can be operated by one person. All documentation is fully recorded in accordance with 21 CFR Part 11 requirements.
包括物料處理和質量控制在內,標準的手動分裝流程需要兩到三名操作員,而自動分裝機只需一人即可操作。所有文件均按照21 CFR Part 11的要求完整記錄。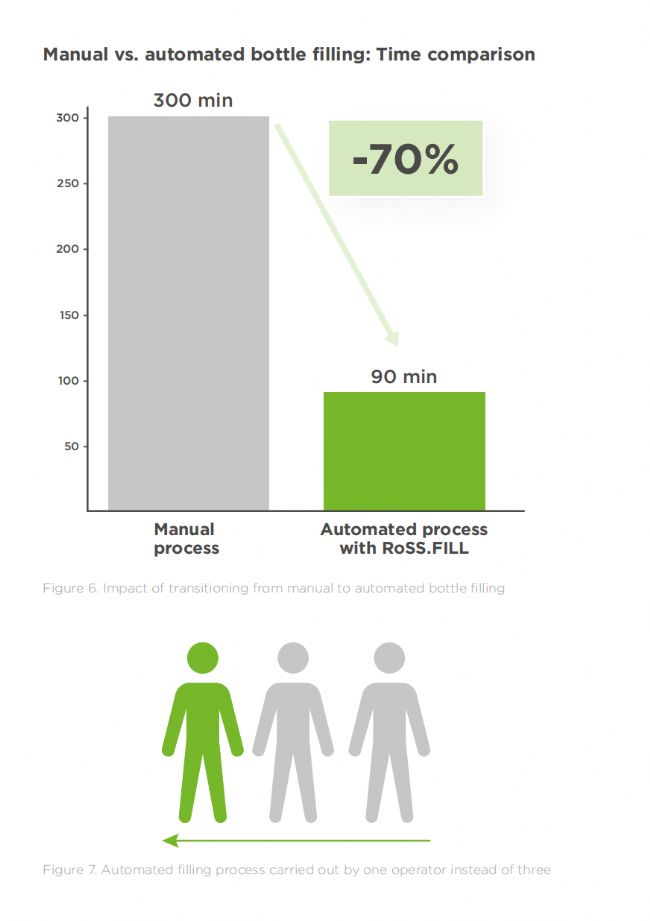
The time saved in filling bottles and bags in parallel increases significantly when relying on automatic filling. This is especially true with modular systems, which enable both containers to be filled using one system.
采用自動分裝技術,可顯著節省同時分裝瓶子和袋子的時間。模塊化系統尤其如此,它允許使用一個系統同時分裝兩個容器。

Additional benefits with closed bottle filler 封閉式分裝機的額外優勢
Aseptically closed system: Using a closed system significantly reduces the risk of human error and exposure to bioburden, especially when working with sensitive biologics, by minimizing open transfers and operator interaction. 無菌密閉系統:使用密閉系統可顯著降低人為失誤和生物負載暴露的風險,尤其是在處理敏感生物制劑時,因為它可以最大限度地減少開放式轉移和操作員互動。
Accuracy: Filling processes can be tailored to meet strict customer requirements – such as ±100g for large bags an ±10g for bottles – ensuring consistent and compliant output. 準確性:分裝流程可根據嚴格的客戶要求進行定制,例如大袋±100克,瓶裝±10克,從而確保輸出的一致性和合規性。
Recovery rate: The system enables maximum recovery rates of any drug substance or drug product, minimizing waste and maximizing yield. 回收率:該系統可提高任何原料藥或藥品的回收率,從而最大限度地減少浪費并提高產量。
GMP compliance: Integrated software solutions that are fully compliant with 21 CFR Part 11, cGMP, and Annex 1 regulations allow supervisor-level control. These features support traceability, audit readiness, and overall process reliability. Together, they contribute to a robust and quality-driven manufacturing environment. 符合GMP標準:集成的軟件解決方案符合21 CFR第11部分、cGMP和附錄1法規,可實現主管級控制。這些功能支持可追溯性、審計準備度和整體流程可靠性。它們共同構成了強大且以質量為導向的生產環境。
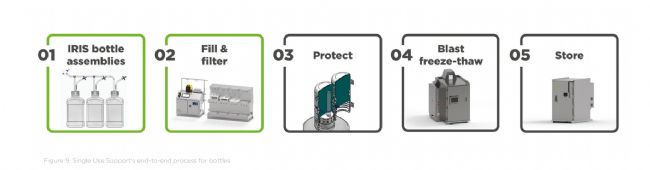
Integrated into the end-to-end bottle process: Like with single-use bags, Single Use Support offers complete solutions for the entire fluid and cold chain management of bottles. These include: 集成到端到端瓶裝流程中:與一次性包裝袋一樣,一次性支持系統為整個瓶裝液體和冷鏈管理提供完整的解決方案。這些解決方案包括:
IRIS Bottle Assemblies: Single-use tubing and manifold assemblies developed for your fluid transfer processes in biopharma processes. IRIS 瓶組件:專為生物制藥工藝中的流體輸送流程而開發的一次性管路和歧管組件。
Bottle RoSS®: Protects tubing assemblies and bottle caps during freezing to ensure product integrity. RoSS® 瓶:在冷凍過程中保護管路組件和瓶蓋,確保產品完整性。
RoSS.BLST: GMP-compliant system for blast freezing & thawing of drug substances – suitable for any primary packaging, including bottles. RoSS.BLST:符合 GMP 標準的藥物速凍和解凍系統,適用于任何初級包裝,包括瓶裝。
lRoSS.ULTF: Ultra-low temperature storage freezer for frozen drug substances in any primary packaging, maintaining stable temperatures down to -80°C
RoSS.ULTF:超低溫冷凍柜,適用于任何初級包裝的冷凍藥物,可在低至 -80°C 的溫度下保持穩定溫度。